Human Pharmacology of Oral DMT Plus Harmine
Copyright © 1997, Jonathan Ott
All Rights Reserved / Reproduced by Permission of Jonathan Ott
(Original Publication: Yearbook for Ethnomedicine 1997/98)
Abstract
A summary is presented of human self-experiments or psychonautic bioassays of pharmahuasca-capsules containing crystalline N,N-dimethyltryptamine (DMT) plus harmine, as well as combinations of other psychoactive tryptamines with other ß-carbolines. The 1967 HOLMSTEDT-LINDGREN hypothesis of the ayahuasca effect-oral psychoactivity of DMT consequent to monoamine-oxidase [MAO] inhibition from concomitant ingestion of ß -carbolines- has been confirmed by 8 self-experimenters. Results of a total of some 70 bioassays are summarized and the literature on this subject is reviewed. Discussion of ayahuasca analogues (anahuasca) focuses on the contemporary non-traditional use of jurema preta (Mimosa tenuiflora) and the ethnobotany and human pharmacology of traditional vinho da jurema is also reviewed [with 94 references and 1Table].
A 1967 analysis of a half-dozen South American snuffs used in shamanic healing by the Tucano, Waiká , Araraibo, Piaroa and Surára Indians, showed all but one of the powders to contain tryptamines, mainly 5-methoxy-N,N-dimethyltryptamine [5-MeO-DMT] and secondarily N,N-dimethyltryptamine [DMT] (HOLMSTEDT & LINDGREN 1967). However, a paricá snuff of the Piaroa Indians of the Venezuelan Orinoco region contained tryptamines–5-OH-DMT [bufotenine], DMT and 5MeO-DMT -together with the ß-carboline alkaloid harmine, while an epéna snuff of the Surára Indians contained only ß-carbolines, which had previously been reported in the same Surára epéna (BERNAUER 1964) and in a Tucano Indian sample of paricá snuff (BIOCCA et al. 1964). Since bufotenine and DMT appeared to be non-psychoactive intranasally (TURNER & MERLIS 1959), HOLMSTEDT and LINDGREN commented:
The occurrence of both tryptamines and ß-carbolines in the South American snuffs is pharmacologically interesting. The ß-carbolines are monoarnine-oxidase inhibitors, and could potentiate the action of the simple indoles. The combination of ß-carbolines and tryptamines would thus be advantageous. (1967: 365)
The following year, HOLMSTEDT & LINDGREN, in collaboration with AGURELL, found DMT in leaves of Diplopterys cabrerana (Cuatrecasas) Gates (Malpighiaceæ] used as an admixture to ayahuasca or yajé by Ecuadorian Kofan Indians (AGLTRELL et al. 1968), a finding replicated by DER MARDEROSIAN and colleagues (DER MARDEROSIAN et al, 1968). Ayahuasca is a pan-Amazonian complex of shamanic potions based on aqueous infusions of the stem of the ayahuasca liana, Banisteriopsis caapi (Spruce ex Griseb.) Morton [Malpighiaceæ], to which may be added visionary, stimulant or curative admixture-pl ants, some 100 of which have been identified (OTT 1993, 1994, 1995c, 1999). Three years earlier, JACQUES POISSON had isolated DMT from dried leaves of D. cabrerana [in all three of these reports the synonym Banisteriopsis rusbyana (Niedenzu) Morton was used] added to natem[a] or ayahuasca by Ecuadorian Shuar Indians (POISSON 1965). Later research documented widespread use of DMT-rich leaves of Psychotria viridis Ruiz et Pavan [Rubiaceæl in ayahuasca potions (OTT 1994, 1999). HOLMSTEDT, LINDGREN and AGURELL extended the earlier observation regarding the snuffs to ayahuasca, noting: “The combination in yajé of monoamine oxidase inhibiting harman alkaloids with N,N-dimethyltryptamine might result in specific pharmacological effects” (AGURELL et al. 1968: 148), an observation echoed by the DER MARDEROSIAN group (1968: 146).
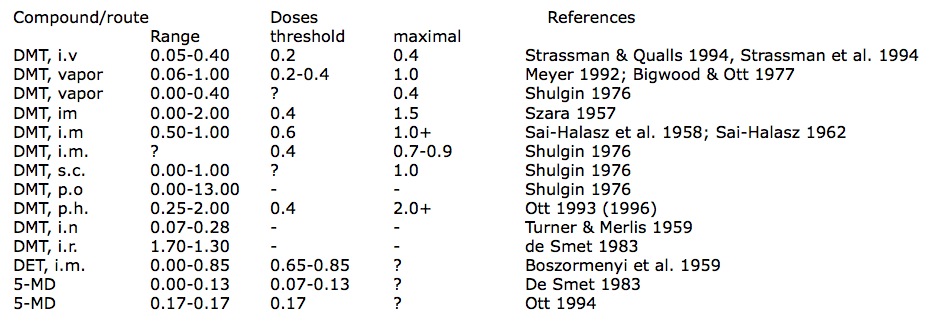
This hypothesis of DMT/ß-carboline synergy was proposed to account for presumed oral activity of DMT in ayahuasca potions. Although this idea was first suggested in relation to the snuffs, this has been all but forgotten, and there have been only rudimentary attempts to model the psychoactivity of the snuffs with pure compounds, although such studies are underway and will be reported in due course. For DMT, first synthesized in 1931 (MANSKE 193 1) and first isolated from Anadenanthera peregrina (L.) Speg. [Leguminosæ] seeds used to prepare cohoba snuff 24 years later (FISH et al. 1955), was found to be inactive orally, in doses as high as 1.0 gram (ca. 13.0 mg/kg; SHULGIN 1976), although it was dramatically psychoactive via intramuscular [i.m.] injection in doses of 30-150 mg (0.4-2.0 mg/kg; SZARA 1957), seems to be quite as active when inhaled as vaporized freebase (0.4-0.5 mg/kg highly active; OTT 1993), and more active still when injected intravenously (i.v.; 0.2-0.4 mg/kg ‘hallucinogenic’; STRASSMAN &QUALLS 1994; STRASSMAN et al. 1994). According to the ingenious HOLMSTEDT-LINDGREN hypothesis, the ß-carbolines present in ayahuasca potions were serving to inhibit the catabolic enzyme monoamine oxidase [MAO] -which would normally metabolize oral DMT before it could get from the digestive system to the brain – so allowing the DMT also present in the ayahuasca potions to be absorbed and transported in the circulation to the brain, there evoking visionary, psychotropic effects.
The HOLMSTEDT-LINDGREN theory — what we might call the ayahuasca effect-won wide acceptance in the literature, for it neatly explained the visionary effects of ayahuasca, that could hardly have been due to the ß-carbolines alone, which elicit rather sedative, Valium® [diazepam] – like psychoactivity, and have a high threshold for oral activity, 8.0 mg/kg in the case of harmine, the main alkaloid of ayahuasca plants and potions (NARANJO 1967). Plants rich in ß-carbolines have found world-wide use as sedatives, a property experimentally verified (MONARDES 1990; MOORE 1989; OGA et al. 1984; SPERONI & MINGHETTI 1988). On the other hand, 5 reported analyses of 17 ayahuasca potions showed an average of 175 mg 0-carbolines per dose [range: 20-441 mg/dose; generally 3 parts harmine to I part d-leptaflorine (R-1,2,3,4-tetrahydroharmine), with traces of harmaline (3,4-dihydroharmine)], which would amount to just over 2 mg/kg [racemic leptaflorine was found to be even less active than harmine, with an oral threshold of 12 mg/kg] (DER MARDEROSIAN et al. 1970; LIWSZYC et al. 1992; McKENNA et al. 1984, 1998; NARANJO 1967; RIVIER & LINDGREN 1972). Since the ß-carbolines per se could not explain the legendary psychoptic activity of the jungle ambrosia, this had to be due to its DMT-content, which amounted to an average of 29 mg/ dose in the 17 potions analyzed [range: 25-36 mg/dose]. Accordingly, the HOLMSTEDT-LINDGREN theory won the day (see OTT 1999, for details and analysis of the phytochernistry of ayahuasca plants and potions, summarized in Tables 11-A, 11-B and 11-C; pp. 36, 38 and 391.
Nevertheless this well-accepted theory had not been tested, either in vitro or in vivo, and remained nothing but a logical explanation of quite scanty phytochemical and pharmacological data. Sixteen years passed before the group of McKENNA showed that two Peruvian ayahuasca samples were ‘extremely effective’ MAOinhibitors in vitro, in a “rat-liver cytosol fraction,” as was an ‘ayahuasca analogue,’ a solution of a mixture of 69% harmine, 26% leptafforine [probably racemic) and 4.6% harmaline, mimicking proportions that had been found in ayahuasca potions (MCKENNA et al. 1984: 218). SO AYAHUASCA was decidedly an MAO-inhibitor, but it remained to be seen whether the sum total of all ß-carbolines present in a typical dose of the potion, 175 mg, could render psychoactive, in a human subject, the 25-36 mg [average: 29 mg] of DMT also present. Only psychonautic bioassays, known as the ‘Heffter Technique’ (OTT 1995d) could establish this with certainty, in the alembic of the human brain (MCKENNA et al. 1984).
When I began to investigate this question in 1990, 1 was able to build on the rudimentary experiments of Bigwood and ‘Gracie and Zarkov.’ Bigwood made a single bioassay of pharmahuasca-a capsule containing 100 mg each of DMT free-base [ 1. 16 mg/kg] and harmaline hydrochloride [= 86 mg free-base; 1.0 mg/ kg], noting: “DMT-like hallucinations … very similar to … a DMT- and harmaline-containing ayahuasca brew that I had previously experimented with” (BIGWOOD 1978). While this single experiment seemingly confirmed the HOLMSTED -TLINDGREN theory, nonetheless it was conducted with some 3-4 times the amount of DMT found in typical doses of ayahuasca, and the ß-carboline chosen, harmaline, “does not contribute significantly” to the pharmacology of the potions (McKENNA et al. 1984: 221). Subsequent ‘underground’ experiments by GRACIE & ZARKOV found DMT active orally in combination with aqueous infusions of ßcarboline-rich seeds of Peganum harmala L. [Zygophyllaceæ; traditionally used as hypnotics; KIRTIKAR et al. 19351, with a threshold level of 20 mg DMT; 30 or 40 mg being a preferred dose (GRAcIE & ZARKOV 1986). Taken together, these pioneering experiments provided tentative, albeit fragmentary, confirmation of the hypothesized ayahuasca effect, but it seemed to me desirable to conduct more systematic psychonautic bioassays of pharmahuasca using measured amounts of both pure DMT and ß-carbolines. Accordingly, for such psychonautic bioassays, I isolated and purified DMT [as free-base, mp 45,’ thin-layer chromatographic (TLC) comparison with reference sample] from roots of Desmanthus illinoensis (Michaux) MacM. [Leguminosæl, and harmine [as the hydrochloride salt, mp 262,’ TLC comparison with authentic reference] from seeds of Peganum harmala, using standard alkaloid-purification techniques as outlined in the literature (McKENNA et al. 1984; MANSKE 1952). Both plants were obtained commercially on the U.S. herbal market. All bioassays were conducted outside of the United States, with standard ‘double-conscious’ procedure, as described by SHULGIN & SHULGIN (199 1: XXVII). ‘Double-conscious,’ a term introduced by GORDON ALLES, means simply that the human-bioassay subject be informed both regarding the identity [as well as the dosage] of the drug being tested, and also as to the nature of the effects which might be anticipated.
In a total of some three dozen experiments [most of which are detailed in OTT 1994], I was able to confirm in vivo the HOLMSTEDT-LINDGREN ayahuasca effect, in my own body. I found that DMT was indeed rendered psychoactive orally in combination with harmine hydrochloride taken simultaneously in a single gelatine-capsule. Starting with quantities near the lowest levels found in ayahuasca portions [20 mg DMT and 40 mg harmine], I systematically tested increasing doses. I found 120 mg of harmine [expressed as the free-base; 1.5 mg/kgl to be the threshold for the ayahuasca effect, whereas in a control experiment with this amount absent DMT, barely-perceptible sedative effects resulted- harmine hydrochloride has been characterized as a ‘stupefying’ agent (FONT QUER 1993: 424). Although I could feet 20 mg DMT [0.25 mg/kgl combined with 120 mg harmine, for me the visionary or psychoptic threshold-level was 30 mg DMT [0.38 mg/kg]. I have tested doses as high as 160 mg DMT [2.0 mg/kgl, experiencing progressively more intense psychotropic effects, but always with the same approximate pharmacodynamics, quite similar to what I have enjoyed with genuine Amazonian ayahuasca potions in Brasil, Ecuador and Perú – 45 minutes to an hour incubation period; the effects quickly building to a peak by 1: 15 and maintaining a plateau for 45 minutes to an hour; followed by about an hour of diminishing effects; the experience usually all but over around the 3 hour point. In no case have I ever experienced nausea in pharmahuasca experiments, although I have weathered nausea and episodes of vomiting provoked by genuine ayahuasca in Amazonia. In any case, I generally eat little or nothing on the day of ingestion. During the experimental series, I always allowed roughly a minimum of a week to elapse between the individual experiments.
I have been able to extend these observations, based on the experiences of eight psychonauts in all, involving a total of about 70 self-experiments. As some of the experimenters wished to remain anonymous, I will merely cite the sole published account and one personal communication (CALLAWAY 1992; MARKUS 1989). In all cases ‘double-conscious’ self-experiments were involved; in no case were the compounds administered to anybody else; and no non-human animal-experimentation of any kind was conducted.
It was found that both harmaline and 6-methoxy-harmalan (MARKUS 1989) could substitute for harmine in pharmahuasca, at approximately commensurate doses-CALLAWAY found 70 mg harmaline [as free-base; 1.2 mg/kg] to activate tryptamines in pharmahuasca, close to the level BIGWOOD had found active [1.0 mg/kg] (BIGWOOD 1978; CALLAWAY 1992). Another psychonaut found 175 mg harmaline hydrochloride [146 mg base; 2.25 mg/kg] alone to be a mild sedative. Chemical analysis showed the aged commercial sample of harmaline used had partially oxidized to harmine, being in reality a mixture of some two thirds harmaline, one-third harmine (SHULGIN 1993). Three different dose-levels of this ‘harmaline’ were then tested in combinations with relatively high doses of tryptamines. Whereas 50 mg [43 mg base; 0.66 mg/kgl was not effective as tryptamine-activator, doses of 100 mg [86 mg base; 1.32 mg/kgj and 150 mg [ 130 mg base; 2.0 mg/kg] definitely were. Thus it would appear that for harmaline, too, there is a threshold for activity in pharmahuasca, somewhere around 0.75 – 1.0 mg/kg. Doses of 6-methoxy-harmalan found to be effective were not divulged to us in the sketchy second-hand report (MARKUS 1989).
CALLAWAY found 10 mg of 5-methoxy-N,N-dimethyltryptamine [5-MeODMT] to be psychoactive in pharmahuasca [expressed as free-base; 0. 17 mg/kg] (CALLAWAY 1992); MARKUS also found this compound psychoactive but we know not the dose (MARKUS 1989). Thus it would appear that 5-MeO-DMT is several times as active as DMT in pharmahuasca; mirroring the higher activity of this compound by other routes. SHULGIN found 5-10 mg doses psychoactive by inhaling the vapor of the free-base (0.07-0.13 mg/kg; SHULGIN 1970,1983). The artificial compound N,N-diethyltryptamine [DET or T-91 was likewise found to be psychoactive orally in pharmahuasca capsules by two psychonauts, who employed doses of 60 mg free-base [0.7 mg/kg1 and 150 mg free-base [2.3 mg/kg] respectively. The latter quantity was characterised as “definitely an overdosage.” We lack enough data to speculate on oral threshold-levels of DET -it is likely this compound is roughly equipotent with DMT in pharmahuasca, much as it is via intramuscular injection. SZARA found 60 mg DET 10.8 mg/kg] psychoactive i.m. (SZARA 1957); the BÖSZÖRMENYI group found it active in doses of from 0.650.85 mg/kg i.m. (BOSZORMENYI et a]. 1959). A recent book (SHULGIN & SHULGIN 1997) gives full details of the chemistry and human pharmacology of the ß-carbolines and tryptamines discussed here, along with some further pharmahuasca data. Whereas 20 and 50 mg of harmaline were insufficient orally to activate 55 and 60 mg of DMT respectively; 80, 100, 150 and 150 mg harmaline did activate 40, 120, 35 and 80 mg DMT respectively. Moreover, 70, 80 and 150 mg harmaline sufficed orally to activate 10, 10 and 25 mg of 5-MeO-DMT respectively. On the other hand, it was noted that harmine [as HCI? 141 mg=120 mg base] could activate 3540 mg DMT orally, in doses in the 140-190 mg range; whereas doses of 120-140 mg harmine were ineffective when taken with 30 mg of DMT.
It has lately been alleged that the ayahuasca effect constitutes ‘potentiation’ of tryptamines by ß-carbolines, as originally suggested with regard to the snuffs (HOLMSTEDT & LINDGREN 1967). In a review article, for example, CALLAWAY noted: “It is well known that ßCs potentiate the activity of methylated tryptamines” (CALLAWAY 1995: 25). However, orally-active DMT in pharmahuasca seems to be weaker than via other routes of administration. It would appear that the descending order of potency via distinct routes is: i.v. injection > inhalation of vapor > i.m. injection > subcutaneous injection > orally in pharmahuasca. Intravenous injection as the fumarate salt appears to be the most effective route; 0.2-0.4 mg/kg was described as ‘hallucinogenic,’ with the higher quantity seemingly representing the maximum effects of the drug (STRASSMAN & QUALLS 1994: 86). Inhalation of the vaporized free-base has a threshold of activity in the 0.2-0.4 mg/kg range (BIGWOOD & OTT 1977), and 40-50 mg was described as a ‘large dose’ (0.5-0.7 mg/kg; MEYER 1992: 154); whereas SHULGIN noted 30 mg (0.4 mg/ kg) evoked a “complete psychedelic experience” (SHULGIN 1976: 167). While psychoactivity was observed with intramuscular injection of 30 mg or 0.4 mg/kg [as hydrochloride salt; this dose was misstated as 0.2 mg/kgl, 0.7-1.0 mg/kg was described as the ‘optimum’ i.m. dose (SZkRA 1957: 461); and experienced users found 1.0 mglkg i.m. (as fumarate salt) “significantly less … hallucinogenic than … previous experience with the smoked [sic] drug” (STRASSMAN & QUALLS 1994: 86). Another researcher characterized the i.m. dose-range as 0.75-1.0 mg/ kg, fixing the threshold level at 0.60 mg/kg: “there are no symptoms at all on administering only 0.50-0.55 mg/kg” (SAI-HALASZ 1962: 137; SAI-HALASZ et al. 1958); although SHULGIN pegged an “abrupt threshold of activity” at 30 mg (0.4 mg/kg) and estimated 50-70 mg i.m. (0.7-0.9 mg/kg) provoked the “complete psychedelic experience.” SHULGIN gave 75 mg (1.0 mg/kg) as the equivalent ‘complete’ dose via subcutaneous injection (SHULGIN 1976: 167). We have seen that the oral DMT-threshold in pharmahuasca is about 0. 3-0.4 mg/kg, and I would estimate that maximum effects would require doses between 1.5-2.0 mg/kg, perhaps more. Intranasal DMT free-base was inactive in doses of 5-20 mg (0.07-0.28 mg/kg; TURNER & MERLIS 1959); likewise inactive were doses of up to 125 mg DMT intrarectally (as a solution of 185 mg bioxalate salt; 1.7 mg/kg; DE SMET 1983). These psychonautic bioassay data are summarized in Table 1.
While the ß-carbolines clearly render DMT active orally, we can hardly characterise this as potentiation. Indeed, it was demonstrated more than 30 years ago that the artificial MAO-inhibitor iproniazid markedly inhibited psychoactive effects of DMT. In subjects given 0.35-0.83 mg/kg DMT i.m.,greatly reduced psychoactivity was experienced when the injections were repeated two days after having received 100 mg iproniazid daily for four days: “the DMT psychosis [sic] … was less pronounced: there were illusions and hallucinations, but without colours, or only with a few of them, and only having the eyes closed” (SAI-HALASZ 1963: 386). The following year, pretreatment with the MAO-inhibitor isocarboxazide “very markedly attenuated” or entirely blocked effects of oral LSD-tartrate (subjects each received LSD, 40 and 75 mcg; then both doses after 2 weeks of isocarboxazide, 30 mg/day; both again after 5 weeks of isocaboxazide treatment; RESNICK et al. 1964: 1211). The MAOI nialamide also ‘blocked’ effects of LSD (GROF & DYTRYCH 1965). A survey conducted by researchers at the U.S. National Institute of Mental Health found: “decrease in response to LSD… in those people who had been taking an MAO inhibitor” (BONSON 1994: 9; BONSON et al. 1996). It is interesting to note that in a single experiment I found pretreatment with isocarboxazide [Marplang], 3 doses of 10 mg in a single day, to render psychoactive 30 mg DMT free-base ingested an hour after the final dose of this artificial MAOinhibitor (OTT 1994,1999), and the reversible inhibitor of monoamine oxidase, moclobemide, at 75-300 mg oral dosage, is also effective at catalyzing the ayahuasca effect in combination with an appropriate oral dose of a visionary tryptamine (TORSTEN 1998).
It would thus appear that the locus of the ayahuasca effect is peripheral and that the MAO-inhibitors which catalyze oral activity of DMT may exert a sort of DMT blocking effect in the brain. While MAOI can render DMT and other tryptamines orally-active, they appear to render it far less potent than when administered via other routes; serving as activators, but not potentiators. We have seen that long-term, daily administration of medicinal MAO-inhibitors (which theoretically elevates brain-serotonine levels) can partially or completely block the effects of both DMT and LSD. This has been documented experimentally and also in surveys of patients undergoing daily MAOI-therapy. Conversely, the potent serotonine-antagonist methysergide or UNIL-491 (Sanserte had “a very strong potentiating effect” on i.m. DMT (SAI-HALASZ 1962: 138), at oral (1-2 mg) or i.m. (0.5 mg) doses well below its own threshold for psychoactivity (4.3 mg; ABRAMSON & ROLLO 1967).
Possible DMT-attenuating actions of MAOI might have some bearing on the fact that DMT orally in pharmahuasca appears to be significantly weaker than via other routes of administration. Strangely, and quite at odds with the limited data at our disposal, ß-carbolines and Peganum harmala seeds have acquired the reputation of all-purpose pan-potentiators of shamanic inebriants, and have been combined by avid ‘basement shamans’ [contemporary, non-traditional aficionados of shamanic inebriants] with psilocine[4-OH-DMT]-containing mushrooms, LSD, even leaves of Salvia divinorum Epling et Jativa [Labiatæl, the visionary principle of which, the diterpenoid salvinorin A, is not even an amine (OTT 1995b, 1996; SIEBERT 1994)! Nevertheless, this ingenious discovery by South American Indians of the ayahuasca effect- conceivably the most sophisticated pharmacognostical discovery ever made in the archaic world-bids fair to revolutionize contemporary, non-traditional entheobotany of visionary shamanic inebriants (OTT 1997).
These results with pharmahuasca have also been extended to so-called ‘ayahuasca analogues’ or anahuasca-the use of non-traditional source plants for either or both tryptamines and ß-carbolines. Since well over 100 plant species in 27 families are known to contain simple ß-carbolines (ALLEN & HOLMSTEDT 1980), some 70 of which contain known MAO-inhibitors (OTT 1993,1994), and some 75 species in 14 families are reported to contain DMT and/or 5-MeO-DMT (OTT 1994; SMFM 1977), there are theoretically several thousand combinations of two plants which could provoke the ayahuasca effect. Indeed, such ayahuasca analogues have lately been made from a variety of plants, although possibly only in Amazonia was the ayahuasca effect exploited in archaic ethnomedicine. Always used as source of ß-carbolines are the seeds of harmel, Syrian rue or Peganum harmala, which are sold worldwide for use as an incense, and the plant is naturalized in North America and Europe (GRACIE & ZARKOV 1986; HASSAN 1967; OTT 1994). Since these seeds contain much higher levels of ß-carbolines than do stems of Banisteriopsis caapi ordinarily used in ayahuasca (2-7% alkaloids, as opposed to an average of 0.45%; McKENNA et al. 1984; OTT 1994; POISSON 1965; RIVIER & LINDGREN 1972; SHAMMA & ABDUL-GHANY 1977), as little as 2-3 grams of harmel seeds will suffice per dose of anahuasca. Although this dose-level serves to activate pure DMT or tryptamines in plants added to the ayahausca analogue, an aqueous infusion of 15 grams of harmel seeds sans additives acted as a Valium®-like sedative, with no visionary effects (OTT 1995c),
Various sources of tryptamines have been used in these analogues, such as roots of Desmanthus illinoensis, Acacia phlebophylla F. von Muell. leaves [Leguminosæ]; and halms of various strains of Phalaris spp. [Gramineæ] (FESTI & SAMORINI 1993[4]; OTT 1995a). Presently the most widely-used source of anahuasca tryptamines is root-bark of Mimosa tenuiflora (Willd.) Poir. [= M. hostilis (Mart.) Benth.; Legurninosæ], source of the ayahuasca-like Brasilian traditional entheogen vinho da jurema.
In 1946, Brasilian microbiologist OSWALDO GONÇALVES DE LIMA reported the continuing shamanic use of ajucá or vinho da jurema among the Pancarurú Indians of Brejo dos Padres near Tacaratú in the valley of the Rio São Francisco in southern Pernambuco. He described the preparation of the potion as a manual, cold, aqueous infusion of pounded root-bark of jurema preta [Mimosa tenuiflora; as M. hostilis], with no additive-plants nor cooking (GONÇALVES DE LIMA 1946). Although thought by SCHULTES to be extinct (SCHULTES & HOFMANN, 1980), we now know that some forms of shamanic ceremony involving vinho da jurema have survived into the 20th century at least among the following indigenous groups in Brasil: Xucurú of Serra de Ararobá in northern Pernambuco (HOHENTHAL 1952); Kariri-Shoko of Colegio near the mouth of the Rio São Francisco which demarcates the Alagoas/Sergipe border (DA MOTA 1987); the Atikum of the Serra do Umã in western Pernambuco (DE AZEVEDo GRONEwALD 1995); the Truká (BATISTA 1995) and numerous other groups scattered sparsely over the immense caatinga of northeastern Brasil (PINTO 1995; TROMBONI 1995). Moreover, in this century, the indigenous jurema ceremony has been adopted symbolically by syncretic Umbandista churches along the Brasilian coast, where jurema preta is not native, centered especially around Alhandra in southern Paraíba (VANDEZANDE 1975).
On the other hand, our only report of contemporary indigenous use of potions prepared from jurema preta root-bark comes from the Atikum of the Serra do Umá region of Pernambuco. Other indigenous groups rather employ one or another type of jurema branca, of which some 10 species have been reported from 4 genera, all but one in the family Leguminosæ: Acacia jarnesiana Willd. (VANDEZANDE 1975); A. piauhyensis Benth. (LEMOS DE ARRUDA CAMARGO 1988); Mimosa burgonia Aubl. (DE ANDRADE MELLO 1955 [as jurema marginada]) M. pudica L. (PINTO 1995); M. verrucosa Benth. (DA MOTA 1987 [as jurema mansal; LEMOS DE ARRUDA CAMARGO 1988; SANGIRARDI 1983); Pithecellobium acacioides Ducke; P. diversifolium Benth.; P. dumosum Benth.; P. tortum Mart. (BATISTA 1995; DE ANDRADE MELLO 1955; LEMOS DE ARRUDA CAMARGO 1988; SANGIRARDI 1983); and Vitex agnus-castus L. [Verbenaceæl (DA MOTA 1987).
It has been reported that Mimosa verrucosa contains DMT, but there is nothing in the chemical literature to support this assertion, and in fact none of these jurema branca species is known to contain visionary tryptamines, although several other species of Acacia do contain DMT and/or 5-MeO-DMT (OTT 1994,1999), whereas bracatinga or Mimosa scabrella Benth. contains low levels [0.03%] of DMT in stem-bark [root-bark untested]. A common source of fuel-wood in southeastern Brasil, this species is known especially for “honey of bracatinga, used as a digestive stimulant and for circulatory problems” (DE MORAES et al. 1990). For a discussion of phytotoxins [especially psychoactive] in honeys, see my recent review article (OTT 1998a).
The taxonomy of jurema preta was recently systematized; its distribution extending from the vast Brasilian caatinga northward to the state of Oaxaca in southern Mexico (BARNEBY 1991). In Mexico the stem-bark is a well-known ethnomedicine, tepescohuite, applied topically for burns and as a vulnerary, but there is no evidence the ancient Mesoamericans exploited the entheogenic virtues of jurema preta (GRETHER 1988). Mexican material of tepescohuite stem -bark was shown to contain low levels [0.03%] of DMT, but there are no published analyses of corresponding root-bark, although bioassays suggest it contains at least 1.0% DMT (MECKES-LOZOYA et al. 1990; OTT 1994,1999).
GONÇALVES DE LIMA reported the isolation of 0.51% of an alkaloid he called nigerina from root-bark of Mimosa tenuiflora collected in Arcoverde, Pernambuco, giving the melting-point as 45.8-46.8’C and the empirical formula C I 3HqNO (GONÇALVES DE LIMA 1946). Nine years later, 0.98% nigerina was again isolated frorn Jurema preta root-bark also collected in Arcoverde (DE MELO 1955). In 1959, GONÇALVES DE LIMA supplied jurema preta root-material to researchers at a U.S. pharmaceutical company, who isolated 0.57% DMT [m.p. 48-49’C, C12 H16 N21 (PACHTER et a]. 1959). It is now thought that nigerina was an impure form of DMT, perhaps contaminated with DMT-N-oxide [readily generated from DMT under isolation conditions] and possibly other compounds. Assuming that GONÇALVES DE LIMA’s nigerina consisted at least partially of DMT, his would represent the first finding of DMT [originally synthesized in 1931 (MANSKE 193 1)] as a natural product, although priority must go to M.S. FISH, who first isolated DMT from Anadenanthera peregrina seeds and pods (FISH et al. 1955).
There are no published analyses of vinho da jurema potions, but a 1983 collaboration between Karolinska Institutet and Universidade Federal do Paraiba in João Pessoa led to two independent analyses of jurema preta potions obtained from an Umbandista juremeira in Alhandra, Paraíba. The first, dated 12 November 1983, was analyzed in Sweden and reported to contain 1-10 mg/mI DMT; while a more precise quantitative analysis in João Pessoa of a potion said to be “identical to the sample taken to Karolinska” but dated 5 December 1983, found 7.46 mg/ml DMT, with the source root-bark containing 11% DMT (HOLMSTEDT 1983; SANCHEZ LEMUS 1984)! The Brasilian group found DMT also in a jurema branca sample from Alhandra, unfortunately unidentified. Thus we have a range of reported DMT concentration in jurema preta root-bark from 1-11% [since PACHTER’s group isolated 0.57% DMT, we can assume a total content perhaps twice as high]; as compared to 0.00-0.66% DMT in reported analyses [12 samples] of Psychotria viridis or chacruna leaf, chief source of tryptamines for ayahuasca potions. Even taking the highest DMT level found in chacruna, jurema preta is 1.5-16.5 times richer in DMT! As for the potions, we have no data on amounts of vinho da jurema typically consumed, but 7.46 mg/ml DMT would correspond to 0.45-1.64 g DMT per dose, taking the range of 60-220 ml reported for typical doses of ayahuasca, analysis of which found 25 mg[220 ml]-36 mg[60 ml] DMT/dose, meaning that VINHO DA JUREMA may be 12.5-65 times higher in DMT than ayahuasca (OTT 1994, 1999)! The vinho da jurema potions analyzed were said to be thick residues or concentrates; even allowing for a 10-fold concentration prior to analysis, vinho da jurema remains 1.25-6.5 times higher in DMT than ayahuasca. Inasmuch as the 36 mg[60 ml] DMT in ayahuasca doses was from Pucallpa and Tarapoto, Perú, where the brews are also considerably concentrated, we can assume the vinho da jurema analyzed to be at least 2.5-3.0 times higher in DMT than typical ayahuasca.
GONÇALVES DE LIMA and HOHENTHAL both described the formation of foam atop the potions when the beaten jurema preta root-bark was hand-squeezed in cold water, and analyses of stem-bark of Mexican tepescohuite jurema preta have found several novel triterpenoid saponins which could explain this phenomenon (ANTON et al. 1993; JIANG et al. 1991 a, 1991 b). Novel chalcone compounds called kukulkanins have also been isolated from branches of Mexican tepescohuite (DOMINGUEZ 1989).
Since the ayahuasca effect depends on presence of ß-carboline alkaloids from Banisteriopsis spp. or other plants, which render DMT orally-active by inhibiting MAO, there has been speculation concerning a lost or missing ingredient to vinho da jurema, or regarding purported content of ß-carbolines in jurema preta. However, no ß-carbolines were found by HOLMSTEDT or SANCHEZ LEMUS, nor in recent unpublished analyses of Mexican root-bark of M..tenuiflora (CALLAWAY 1998). In the Serra do Umã, where use of jurema preta potions survives, it was noted that juice of maracuja was consumed freely during the jurema ceremony (DE AZEVEDO GRUNEWALD 1995). Since maracuja juice, from Passiflora spp. [Passifloraceae], contains ß-carbolines (LUTOMSKI et al. 1975), it was suggested such might account for oral activity of DMT in the potions. However, the Passiflora spp. contain especially harman [or passiflorinel, which was found not to be effective as a human MAOI in pharmahuasca bioassays (OTT 1994,1999). None of the scanty ethnographic reports support the notion of a lost or missing additive-plant, although they do stress prodigal use of smoked tobacco as adjunct to jurema ingestion. Recently it was found that tobacco-smokers show 40% inhibition of cerebral MAOB (FOWLER et al. 1996), which would seem to be insufficient of itself to render DMT active orally, although it could be a contributing factor. The MAOI effect of smoked tobacco is not understood chemically, but low levels of ß-carbolines are known from tobacco-smoke (JANIGER & DOBKIN DE RIOS 1976).
Whereas a potion prepared from 10 g Mimosa tenuiflora root-bark [3 times extracted in acidified hot water] was inactive as to visionary effects, I recently found psychoptic properties in a potion prepared from 25 g jurema preta root-bark. The potion was prepared by the traditional method, simply squeezing the beaten rootbark in cold water, with no additives [2 times, 125 ml water each time]. The vinho da jurema gave DMT-like effects commencing somewhat sooner [20 min.] and lasting less time [> 2 h.] than is typical for me with ayahuasca or pharmahuasca [ca. 45 min.; lasting 2 h.+] (OTT 1998b). As there exist non-ß-carboline MAOI, there is the possibility some unknown MAOI compound exists in jurema preta. On the other hand, preliminary analyses of Mexican root-bark suggest that, apart from free DMT, jurema preta contains DMT bound or complexed to larger molecules which might protect against deamination by MAO and allow transport to the brain [where free DMT would probably have to be generated by action of another enzyme] (CALLAWAY 1998). In any case, it is evident there is no lost or missing ingredient, and vinho da jurema is potently visionary by itself, prepared in the traditional manner, and assuming an adequate dose. The apparent inactivity of simple Atikurn jurema preta potions is thus likely due to insufficient dosage or perhaps a weak strain, and not to the lack of some lost ingredient. The same may hold true for the reported lack of activity of a Kariri-Shoko jurema mansa potion [Mimosa verrucosa]; or perhaps this species contains no bioavailable DMT (DA MOTA 1987). Only further chemical research linked to human psychonautic bioassays wilt resolve the conundrum of the psychoptic pharmacology of vinho da jurema. Meanwhile, jurema preta root-bark is being sold and widely used in Europe and North America as a visionary substrate for contemporary anahuasca potions.
Table 1
Human Pharmacology of Psychoptic Tryptamines
Pharmahuasca, Anahuasca and Vinho da Jurema
Acknowledgements
I am beholden to Drs. James C. Callaway, Mark S. Donnell, Jochen Gartz, Robert Montgomery, Torsten and Alexander T. Shulgin for discussions and advice. This paper is dedicated to Prof. Richard Evans Schultes of Harvard University, in grateful recognition of his pioneering role in elucidating the botany and ethnopharmacognosy of ayahuasca potions.