by Brian Vastag
Journal of the American Medical Association
Vol. 288 No. 24, pp. 3096-3101,
December 25, 2002
© 2002 American Medical Association.
All rights reserved.
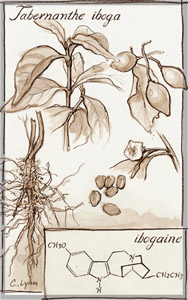 Tabernanthe iboga, the West African source of ibogaine, used by some to treat addiction.
Tabernanthe iboga, the West African source of ibogaine, used by some to treat addiction.
New York — Some drugs are made in laboratories. Others, like penicillin, are discovered by accident. And then there’s ibogaine, a sacramental substance from West Africa that some say interrupts heroin, cocaine, and other addictions. Over the past 40 years, the tale of ibogaine’s flirtation with legitimacy boasts more twists than the roots of Tabernanthe iboga, the shrublike source of ibogaine.
After riding the backpacks of Westerners to the radical 1960s New York City underground, ibogaine rose from a counterculture star to a serious project funded by the National Institutes of Health (NIH). In 1995, after spending several million dollars on laboratory and animal studies, the NIH decided not to pursue ibogaine development. Since then, patent disputes have divided the drug’s champions; a growing network of informal clinics has sprung up; and pharmacologists have discovered that ibogaine works on the brain in a manner unlike that of any other known drug.
(See: Sidebar 1.)
After all this, ibogaine and two of its derivatives appear closer to legitimacy now than ever before. In 1998, a University of Miami Medical Center researcher opened an ibogaine clinic on the Caribbean island of St Kitt’s. Although the US Food and Drug Administration (FDA) had approved human trials with ibogaine, Deborah Mash, PhD, associate professor of neurology and pharmacology at Miami, could not secure funding for a stateside study. Instead, she solicited private investment and won favor from the government of St Kitt’s, where a team of physician counselors and addiction specialists now collect data that Mash hopes will cement support for US trials of ibogaine or its metabolite, noribogaine.
Meanwhile, another pharmacologist, Stanley Glick, MD, PhD, director of the Center for Neuropharmacology and Neuroscience at Albany Medical Center, has painstakingly moved a derivative of ibogaine toward its own clinical trial. After 12 years of basic research on scores of molecular variations on the ibogaine theme, Glick recently forged an agreement that represents his best chance for a clinical trial. Signed in November 2002, the contract obligates investors to raise $5 million within 2 years to fund the first human studies of 18-methoxycoronaridine (18-MC).
But even as ibogaine’s supporters sniff success, they worry that the drug’s origins will continue to stunt its development. “It’s been a continuous battle for respect,” said Glick. “Ibogaine has really become notorious because it didn’t originate in a lab, but in the counterculture.”
Mash is concerned that burgeoning unsanctioned use will compromise years of laboratory and clinical work. “We’ve got this explosion of underground clinics, and I’m scared that everything I work for is going to go right down the toilet,” Mash said in a recent telephone interview. As an endowed, tenured professor, Mash has all the right credentials: a 29-page curriculum vitae listing 155 publications; a history of millions of dollars in federal grants; a spot at the table of several National Institute on Drug Abuse (NIDA) review committees; and a reputation as a brilliant brain scientist.
And yet, Mash feels that ibogaine’s tumultuous history (see sidebar 2) has isolated her. “I’m the only one [doing clinical research],” she said. “I figured, somebody ought to test the damn thing. You know, either it works or it doesn’t.”
SCIENTISTS LOOK INTO USE
In 1999, Kenneth Alper, MD, PhD, assistant professor of psychiatry at New York University School of Medicine, hosted the first serious scientific conference devoted to ibogaine. He and Glick compiled the proceedings into a thick volume (Alkaloids Chem Biol. 2001;56:1-330). In the preface, Geoffrey Cordell, PhD, a pharmacology researcher at the University of Illinois at Chicago, writes that while ibogaine probably “won’t save the world from addiction,” it deserves a “prominent position in the list of anti-addictive strategies” under study.
Animal data support Cordell’s conclusion. Dozens of articles referenced in the conference proceedings report reductions in self-administration of morphine, heroin, cocaine, alcohol, and nicotine in rodents given ibogaine. The effects last from 1 to 5 days, depending on dosage and other variables. Noribogaine and 18-MC produced similar results.
That means the central hurdle for ibogaine’s supporters is amassing compelling human data. While unknowable scores of addicts continue ingesting ibogaine hydrochloride a purified powder — or iboga whole-plant extract containing a dozen or more active alkaloids — few trained researchers witness the events.
“There’s basically one big uncontrolled experiment going on out there,” said Frank Vocci, PhD, head of antiaddiction drug development at NIDA.
Consequently, supporters have had to rely on anecdotal accounts. At a pivotal 1995 NIDA meeting, Howard Lotsof, credited with discovering ibogaine’s purported antiaddictive potential, presented a collection of case reports. He reported that 10 (19%) of 52 treatments led to cessation of heroin or cocaine use for a year or longer; 15 (29%) treatments led to 2 months or less of sobriety. The remaining treatments were followed by sober periods between 2 months and 1 year. Despite Lotsof’s report, the NIDA peer review panel voted nine to four to reject a clinical grant application from Mash.
She regrouped and eventually opened the Healing Visions clinic in St Kitt’s. In 2000, Mash and colleagues published the data from 27 cocaine- or heroin-addicted patients treated at the center (Ann N Y Acad Sci. 2000:914;394-401). The researchers conclude that “self-reported depressive symptoms and craving were significantly decreased” at 1 month after stopping treatment with ibogaine. They also note that ibogaine treatment “decreased participants’ desire and intention to use heroin.”
Mash is now analyzing safety and efficacy data for 257 patients.
SAFETY CONCERNS
At Healing Visions, patients receive what Mash calls “state-of-the-art care,” with round-the-clock monitoring and access to the latest emergency equipment. But individuals who seek out ibogaine in other settings receive no such supervision. “It’s caveat emptor,” said NIDA’s Vocci.
Vocci also said that safety was “not the main concern” at the pivotal 1995 NIDA meeting, which he chaired. However, that review panel did cite safety issues. One reviewer wrote that the drug’s toxicology profile was “less than ideal,” with bradycardia leading the list of worrisome adverse effects.
In fact, between 1989 and 2000, three reports of patients dying after taking ibogaine surfaced, sparking a swirl of questions about the drug’s safety. The first death, of a 40-year-old woman in France, apparently stemmed from preexisting heart disease. A lack of medical information hindered investigations into the other two deaths and led to conflicting conclusions about whether ibogaine was to blame.
In a 1996 radio interview with WBAI in New York City, Mash said that, in the French case, the patient “was very sick, she had a very sick heart and she shouldn’t have been given ibogaine under any circumstances. . . .” And in the second death, “we don’t completely know the mechanism of lethality, but it did appear to be respiratory collapse in this case. The bottom line is that you need to be under medical supervision. . . . Ibogaine is an important drug but it is not to be used outside the medical establishment, not ever, ever, ever.”
Despite Mash’s warnings, unsanctioned ibogaine use appears to be soaring. A sophisticated “underground railroad” of sorts has sprung up in New York, spearheaded by Dana Beal, a long-time marijuana legalization advocate. When heroin- or cocaine-addicted individuals develop an interest in ibogaine, they often call Beal, who acts as intake counselor.
During an interview in his home, the one-time headquarters of the radical 1960s Yipster Times newspaper, Beal said that if he thinks someone is a good candidate for ibogaine, he helps arrange a visit to an informal clinic.
The best known operation, according to Beal, is in the Netherlands at the Amsterdam home of Sara Glatt, who practices various types of alternative medicine. Glatt has treated some 85 people during the last 3 years. When an addicted individual arrives, Glatt asks for a history of heart problems or bad experiences with psychedelic drugs. Judging from that information and the individual’s weight, Glatt provides between 2 g and 6 g of powdered iboga, the whole-plant extract that contains at least a dozen active ingredients in addition to ibogaine.
Whereas Glatt charges upward of $1000 for her services, the newest clinic, in Vancouver, British Columbia, offers free ibogaine. The clinic’s founder, Marc Emery, won 2000 of 140 000 votes in the 2002 Vancouver mayoral election running on a platform of open access to ibogaine. He recently solicited an ibogaine e-mail list for feedback on a proposed treatment regimen.
Lotsof, on the other hand, has already published a rigorous protocol (Lotsof H, Wachtel B. Manual for Ibogaine Therapy: Screening, Safety, Monitoring, and Aftercare, First Revision. Published online. Available at http://www.ibogaine.org/manual.html. Accessed November 26, 2002). In the preface to the first revision, Lotsof and coauthor Boaz Wachtel write that the manual is “intended for lay-healers who have little or no medical experience, but who are nevertheless concerned with patient safety and the outcome of ibogaine treatments.” The manual suggests inclusion and exclusion criteria, ibogaine regimens and doses, and considerations for post-treatment care. A naive physician would likely accept it as a standard medical protocol.
Back in the realm of sanctioned drug development, Glick and Mash are now focused on bringing their respective ibogaine derivatives into clinical trials. “That’s certainly the way to go now,” said Vocci. Alper voiced a similar opinion, saying that he views ibogaine as proof of concept that the best hope for a therapeutic drug lies with ibogaine derivatives. Glick, too, is certain that the FDA will never approve ibogaine. In addition to safety concerns and the drug’s social history, the hallucinogenic effects of ibogaine (see: Sidebar 1) could be problematic.
After NIDA rejected ibogaine clinical trials, both Mash and Glick struck out with the pharmaceutical industry, which has been traditionally cool to antiaddiction drugs. The Pharmaceutical Research and Manufacturers of America (PhRMA) reports that in 1999, for example, its roster of drug giants had 10 antiaddiction agents in clinical trials. The same companies had more than 400 cancer drugs in clinical development. When asked to explain the disparity, Jeff Trewhitt, spokesman for PhRMA, said, “We certainly don’t know a reason, unfortunately.”
But ibogaine researchers and others, including a spokeswoman for the Substance Abuse and Mental Health Services Administration (SAMHSA), say that addiction stigma and low profit potential are keeping companies away.
Whatever the case, the dearth of pharmaceutical and other treatments means that the societal costs of addiction will continue to climb. SAMHSA reports that in 2000, illicit drug addiction cost the United States $160 billion in medical care, lost productivity, and crime and incarceration, up from $117 billion in 1997. Illicit drug addiction is here to stay.
So too, it appears, is ibogaine.
An Odd Drug (Sidebar: 1)
Other hallucinations passed before my eyes burning skulls and faces, the figures of women in black dresses stretching out long white arms toward me from the edges of my vision but when I tried to speak of them, they disappeared. Meanwhile, the iboga was making me sick. I fought back waves of nausea. I wanted to reach the deeper visionary state, but I was also afraid of the drug.
Journalist Daniel Pinchbeck, in Breaking Open the Head: A Psychedelic Journey Into the Heart of Contemporary Shamanism. New York, NY: Broadway Books; 2002.
At low doses, ibogaine is a mild stimulant. At high doses, users report deeply emotional visions, sometimes pleasant, sometimes harrowing. Patrick Kroupa, who credits ibogaine with 3 years of sobriety after 15 years of addiction, said, “It was like dying and going to hell 1000 times.”
Whatever the subjective experience, pharmacologists have spent decades puzzling out the brain effects of ibogaine. Their conclusion: it’s unlike any other known drug. Kenneth Alper, PhD, assistant professor of psychiatry at New York University School of Medicine, said that the drug appears to work on “every neurotransmitter system we know about.” It binds to N-methyl-D-aspartate receptors and u- and k-opioid receptors; all three play prominent roles in current theories of addiction.
Ibogaine also acts as an antidepressant by binding to serotonin transporters, thereby increasing serotonin levels in the nucleus accumbens. Evidence of impact on the dopamine and acetylcholine systems is less compelling, but deserves consideration, said Alper (Alkaloids Chem Biol. 2001;56:2-33).
Most recently, Stanley Glick, MD, PhD, published support for his theory that ibogaine reduces drug-seeking behavior in rodents by blocking a3b4 nicotinic receptors (Eur J Pharmacol. 2002;438:99-105).
Meanwhile, Deborah Mash, PhD, a neuroscientist at University of Miami Medical Center, is convinced that ibogaine is nothing but a short-acting prodrug. It quickly metabolizes into noribogaine, she said, which boasts a half-life so long that she has been unable to measure it. This property, she believes, explains ibogaine’s purported ability to block drug cravings for weeks or months (Alkaloids Chem Biol. 2001;56:79-113).B.V.
A Brief History of Ibogaine (Sidebar: 2)
1885: First published description of religious use of Tabernanthe iboga in Gabon appears in France; it reports that initiates of the Bwiti religion eat rootbark to induce visions and “meet their ancestors.”
1939: Sold in France as a stimulant until 1970.
1962: Howard Lotsof, a 19-year-old from Staten Island, receives ibogaine from an LSD chemist and gives it to 19 other people. He later reports that five of seven heroin and cocaine addicts in this group, including himself, stop illicit drug use for up to 18 months and experience little or no acute withdrawal.
1970: The US Food and Drug Administration (FDA) classifies ibogaine as a Schedule I drug, making it illegal. Belgium also outlaws ibogaine, but today it remains legal in the rest of the world.
1985: Lotsof receives a US patent for use of ibogaine in opioid withdrawal. Additional patents describing ibogaine treatment for cocaine, and other addictions follow.
1989: Ibogaine addiction treatment begins in informal clinics in the Netherlands. By 2002, informal clinics have opened in the United Kingdom, Canada, Slovenia, and Mexico.
1991: After intense pressure from activists, the National Institute on Drug Abuse (NIDA) begins funding preclinical toxicology and other laboratory research on ibogaine. 1993: The FDA approves a US clinical trial of ibogaine sponsored by University of Miami neuroscientist Deborah Mash, PhD.
1995: NIDA review committee rejects funding for Mash’s clinical trial.
1999: Mash opens ibogaine clinic on Caribbean island of St Kitt’s. By late 2002, she has collected safety and efficacy data on 257 addicted patients.
2002: Long-running legal dispute between Lotsof and Mash ends with the University of Miami winning patents for noribogaine, a metabolite of ibogaine. Stanley Glick, MD, PhD, director of the Center for Neuropharmacology and Neuroscience at Albany Medical Center, signs contract to bring ibogaine derivative 18-MC into clinical trials.
–B.V