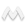Reprinted from Neurobiological Mechanisms of Drugs of Abuse
Volume 914 of the Annals of the New York Academy of Sciences
September 2000
Ibogaine: Complex Pharmacokinetics, Concerns for Safety, and Preliminary Efficacy Measures
DEBORAH C. MASH,[a,b,h]
CRAIG A. KOVERA,[o] JOHN PABLO,[o]
RACHEL F. TYNDALE,[c] FRANK D. ERVIN,[d]
IZBEN C. WILLIAMS,[e]
EDWARD G. SINGLETON,[f] and MANNY MAYOR,[g]
Departments of [a]Neurology, [b]Pharmacology, and [g]Medicine,
University of Miami School of Medicine, Miami, Florida, USA
[c]Centre for Addiction and Mental Health,
University of Toronto, Toronto, Canada
[d]Department of Psychiatry and Human Genetics,
McGill University, Montreal, Canada
[e]Healing Visions Institute for Addiction Recovery, Ltd.,
St. Kitts, West Indies
[f]Behavior Therapy Treatment Research Center,
Johns Hopkins Medical School, Baltimore, Maryland, USA
ABSTRACT: Ibogaine is an indole alkaloid found in the roots of Tabernanthe Iboga (Apocynaceae family), a rain forest shrub that is native to western Africa. Ibogaine is used by indigenous peoples in low doses to combat fatigue, hunger and thirst, and in higher doses as a sacrament in religious rituals. Members of American and European addict self-help groups have claimed that ibogaine promotes long-term drug abstinence from addictive substances, including psychostimulants and opiates. Anecdotal reports attest that a single dose of ibogaine eliminates opiate withdrawal symptoms and reduces drug craving for extended periods of time. The purported efficacy of ibogaine for the treatment of drug dependence may be due in part to an active metabolite. The majority of ibogaine biotransformation proceeds via CYP2D6, including the O-demethylation of ibogaine to 12-hydroxyibogamine (noribogaine). Blood concentration-time effect profiles of ibogaine and noribogaine obtained for individual subjects after single oral dose administrations demonstrate complex pharmacokinetic profiles. Ibogaine has shown preliminary efficacy for opiate detoxification and for short-term stabilization of drug-dependent persons as they prepare to enter substance abuse treatment. We report here that ibogaine significantly decreased craving for cocaine and heroin during inpatient detoxification. Self-reports of depressive symptoms were also significantly lower after ibogaine treatment and at 30 days after program discharge. Because ibogaine is cleared rapidly from the blood, the beneficial aftereffects of the drug on craving and depressed mood may be related to the effects of noribogaine on the central nervous system.
[h]Address for correspondence:
Deborah C. Mash, Ph.D., Department of Neurology (D4-5).
1501 N.W. 9th Avenue, Miami, Florida 33136.
Tel.: (305) 243.5888 / Fax: (305) 243.3649
e-mail: dmash@newssun.miami.edu
INTRODUCTION
Ibogaine is a naturally occurring indole alkaloid derived from the roots of the rain forest shrub Tabernanthe iboga. Ibogaine is used in low doses by indigenous peoples of western Africa to combat fatigue, hunger and thirst, and in higher doses as a sacrament in religious rituals. The use of ibogaine for the treatment of drug dependence has been based on anecdotal reports from American and European addict self-help groups that it decreased the signs of opiate withdrawal and reduced drug craving for cocaine and heroin for extended time periods.1 Although ibogaine has diverse effects on the central nervous system (CNS), the pharmacological targets underlying the physiological and psychological actions of ibogaine are not completely understood. The purported efficacy of ibogaine following single-dose administrations may be due to the formation of an active metabolite.2,3 Ibogaine is O-demethylated to 12-hydroxyibogamine (noribogaine) by the activity of liver enzymes. Noribogaine appears to have a slow clearance rate in humans, suggesting that some of the aftereffects of ibogaine may be due to the actions of the metabolite.3,4
Medications for the treatment of addictions are intended to reduce or eliminate drug use, reduce harm to the patient, and/or modify high risk behaviors.5 Potential drug candidates for the treatment of cocaine and opiate dependence have been considered based on pharmacological approaches. The neurobiological similarities between depression and drug dependence have led to a self-medication hypothesis.6 Based on this association, drug abusers may self-medicate with opioids or psychostimulants in an effort to improve their dysphoric mood. Chronic drug abuse leads to adaptations at neural systems that mediate drive, motivation, and affect.7 Discontinuation of drug use leads to alterations in these motivational systems, placing more incentive value to the abused drug than to nondrug stimuli, thus leading to a loss of control over drug use.8 This hypothesis is supported by clinical observation that drug dependence is characterized by an intense desire to administer the drug to the exclusion of other reinforcement. We report here that single-dose administrations of ibogaine to drug-dependent individuals resulted in fewer self-reports of craving for cocaine and opiates, and significantly improved depressive symptoms. These preliminary observations provide evidence for an improvement in clinical status following detoxification with ibogaine.
METHODS
Subjects were self-referred for inpatient detoxification and met inclusion/exclusion criteria. All individuals were deemed fit and underwent treatment following a physician’s review of the history and physical examination. Participants did not have histories of stroke, epilepsy or axis I psychotic disorders. Results of the electrocardiogram and clinical laboratory testing were within predetermined limits. All subjects signed an informed consent for ibogaine treatment. Participants included 27 treatment-seeking opioid- and cocaine-dependent men (n = 23) and women (n = 4). The mean age was 34.6 +/- 1.9 years old for the opiate group and 37.5 +/- 2.9 years old for the cocaine group. Mean education level was 14.0 +/- 0.5 years. All participants met DSM-IV criteria for cocaine or opioid dependence and had positive urine screens at entry to the study. Individuals participated in a 14-day inpatient study to determine the safety and effectiveness of ibogaine as a potential medication treatment for drug dependence. Participants were assigned to one of three fixed-dose (500, 600, or 800 mg) ibogaine HCl treatments under open-label conditions. Ibogaine and 12-hydroxyibogamine (noribogaine) were measured in whole blood specimens by full-scan electron impact gas chromatography/mass spectrometry (GC/MS) as described previously.2 Subjects were genotyped for the CYP2D6 alleles (3, 4, 5, and wildtype alleles) as described previously.9
On admission, participants were administered the Addiction Severity Index10 and received structured psychiatric evaluations before and after ibogaine treatment (SCID I and II). In cases where the participant’s responses were deemed questionable due to intoxication or withdrawal signs, portions of all interviews were reconducted later, as necessary. Additional information about substance use history, as well as past and current medical condition(s), was gathered and later cross-referenced for accuracy through a separate comprehensive Psychosocial Assessment.
Participants were required to complete a series of standardized self-report instruments relating to mood and craving at three different time points during the study and at one month following discharge from the treatment program. Subjects were asked to provide ratings of their current level of craving for cocaine or opiates using questions from the Heroin (HCQN-29)11 and Cocaine (CCQN-45) Craving Questionnaires.12 Self-reported depressive symptoms were determined by the Beck Depression Inventory.13 Subjects’ scores were subjected to repeated measures analyses of variance with primary drug of abuse (opiates versus cocaine) as the between-subjects factor and treatment phase (pre-ibogaine, post-ibogaine, and discharge) as the within-subjects factor for the total score from the Beck Depression Inventory and the HCQN-29 and CCQN-45 subscales.
RESULTS
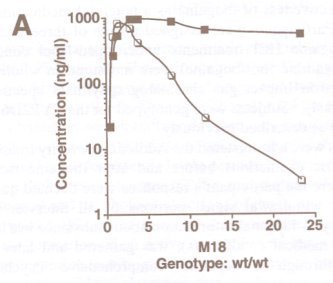
Chart: A
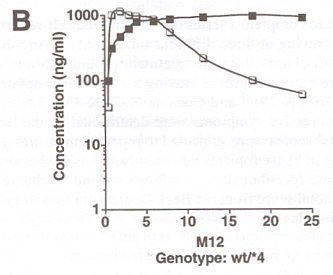
Chart B
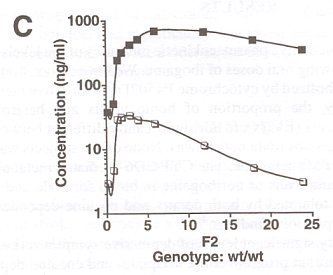
Chart C
FIGURE 1. Pharmacokinetics of ibogaine and noribogaine over the first 24 h after oral doses of ibogaine. Data shown are from representative male (800 mg) and female (500 mg) subjects. Values for parent drug and desmethyl metabolite were measured in whole blood samples at the times indicated.
FIGURE 1 illustrates representative pharmacokinetic measures of the levels of parent drug and metabolite following oral doses of ibogaine. We have demonstrated previously that ibogaine is metabolized by cytochrome P4502D6 to an active metabolite noribogaine.14 In this study, the proportion of homozygous and heterozygous CYP2D6 extensive metabolizers (EM) were not significantly different between opioid- and cocaine-dependent groups (data not shown). None of the subjects was identified as a poor metabolizer (PM) genotype. The CYP-2D6-mediated metabolism of ibogaine resulted in significant levels of noribogaine in blood for male and female subjects. Ibogaine was well tolerated by both opiate- and cocaine-dependent subjects, in agreement with our previous findings.

Table 1
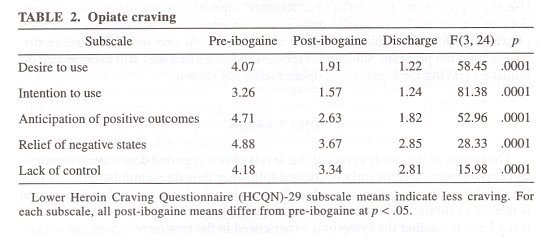
Table 2
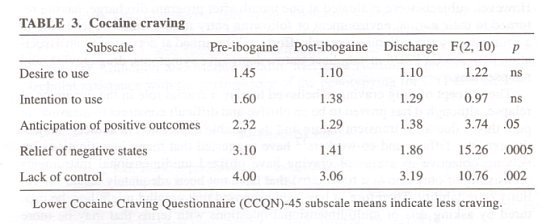
Table 3
Subjects reported clinically significant levels of depressive symptoms (i.e., Beck depression score of 11 or more) at program entry.13 Opiate- and cocaine-dependent subjects did not differ significantly on the Beck depression scores. TABLE 1 summarizes the effects of ibogaine treatment on depressive symptoms following ibogaine detoxification from opiates and cocaine. The results demonstrated lower levels of self-reported depressive symptoms during inpatient ibogaine treatment. Beck depression scores were significantly lower at one month after program discharge as compared to those measured at program entry. This observation suggests a lasting effect of single-dose adminstration of ibogaine on mood and depressive symptoms.
TABLE 3 summarizes the results for selected categories from the HCNQ-29 and CCNQ-45 craving questionnaires. These instruments inquire about specific aspects of drug craving, including urges (category scale Desire to Use), as well as thoughts, about drug of choice or plans to use the drug (category scale, Intention to Use). The instruments incorporate questions about dynamics thought to be important for the reinstatement of drug-taking behavior by referencing the positive reinforcing effects of drugs or the expectation of the outcome from using a drug of choice (category scale, Anticipation of Positive Outcomes), or the alleviation of withdrawal states (category scale, Relief of Negative States). Perceived lack of control over drug use also was included (category scale, Lack of Control), because it is a common feature of substance abuse disorders and is most operative under conditions of active use, relapse, or for subjects at high risk.
Subjects undergoing opiate detoxification reported significantly decreased drug craving for all of the HCQN-29 scales at 36 h post-treatment mark, and mean scores remained significantly decreased at program discharge (TABLE 2). Subjects undergoing cocaine detoxification also reported significantly decreased drug craving at post-treatment and at discharge for three of the five category scales of the CCQN-45. Similar to the results of the HCQN-29, the lower mean scores for Intention to Use at program entry may reflect participants’ inpatient circumstances and motivation for treatment. These results demonstrate an immediate and lasting attenuation of craving while the subject remained in treatment. At one month following discharge from the program, subjects’ responses indicated that they still experienced diminished craving for cocaine and opiates (data not shown).
DISCUSSION
The results of this study revealed that levels of self -reported depressive symptoms and craving were significantly decreased following ibogaine administration. A limitation of the study is that it is based on findings in only 27 subjects; thus, replication is needed in future studies to determine the stability of the findings. Furthermore, it is not known whether the symptoms experienced in the treatment setting are unique. However, subjects were evaluated at one month after program discharge, having returned to their normal environment or following entry to residential sober living in a community setting. Future research efforts will be aimed at determining an association between CYP2D6 metabolizer status, depressive symptoms, drug craving, and relapse rates.
The concept of drug craving is believed to playa crucial role in the dynamics of relapse, although it has proven to be an elusive and difficult construct to measure. In part, this is due to its transient nature and its variable expression in addicts’ subjective reports. Tiffany and co-workers12 have suggested that many investigations involving objective measures of craving have utilized unidimensional instruments (consisting of only one or a few items) that have not been adequately tested for validity or reliability. Therefore, it has been proposed that craving might best be captured by asking sets of multidimensional questions with terms that may be more familiar to addicts and that represent distinct conceptualizations of processes that may lead to craving.12,15 To our knowledge, this study represents the first attempt to confirm ibogaine’s purported therapeutic effects on drug craving in well-characterized cohorts of opiate- or cocaine-dependent subjects.
After treatment with ibogaine, opiate-dependent subjects were less likely to anticipate positive outcomes from heroin (or other opiate) use, less likely to believe that heroin (or opiate) use would relieve withdrawal/dysphoria, and more likely to believe in their control for abstaining or stopping their drug use. Ibogaine treatment also decreased participants’ desire and intention to use heroin. Cocaine-dependent subjects were less likely to anticipate positive outcomes from cocaine use, less likely to believe that cocaine use would relieve withdrawal/dysphoria, and more likely to believe in their control in abstaining or stopping their drug use at post-ibogaine and discharge assessments than at the pre-ibogaine assessment. Treatment did not seem to affect participants’ desire to use cocaine nor their intent to use cocaine, in part because of floor effects at pre-ibogaine assessments and because of the small sample size.
Drug dependence results from distinct but interrelated neurochemical adaptations, which underlie tolerance, sensitization, and withdrawal. The apparent ability of ibogaine to alter drug-taking behavior may be due to combined actions of either the parent drug and/or its active metabolite at key pharmacological targets that modulate the activity of drug-reward circuits.16 Noribogaine is longer lasting and has a unique spectrum of neurochemical activities as compared to the parent compound. Recent studies have suggested that noribogaine’s efficacy as a full û-opioid agonist may explain ibogaine’s ability to block the acute signs of opiate withdrawal and its suppressive effects on morphine self-administration.17 Preclinical evaluation ofnoribogaine’s anti-cocaine medication effects in a rat model of cocaine self-administration demonstrated that noribogaine antagonized cocaine-induced locomotor stimulation and reinforcement.18 Since ibogaine is cleared rapidly from the blood, the extended aftereffects on drug craving, mood, and cognition may be related to the actions of metabolite noribogaine. Medication development of a slow-release formulation of noribogaine for opiates and psycho stimulants dependence deserves further consideration.
ACKNOWLEDGMENTS
This work was supported in part by the Addiction Research Fund. We extend our appreciation to the staff of the Healing Visions Institute for Addiction Recovery, Ltd., St. Kitts, W.I. and Holistic Counseling, Inc., Miami, Florida. We thank the Centre for Addiction and Mental Health. Ms. Ewa Hoffmann provided excellent technial assistance with the performance of the genotypying for CYP2D6 alleles.4
REFERENCES
1. SHEPARD, S.G. 1994. A preliminary investigation of ibogaine: case reports and recommendations for the further study. I. Subst. Abuse Treat. 11: 379-385.
2. HEARN, W. L., I. PABLO, G. HIME & D.C. MASH. 1995. Identificaion and quantitation of ibogaine and an O-demethylated metabolite in brain and biological fluids using gas chromatography/mass spectrometry. I. Anal. Toxicol. 19: 427-434.
3. MASH, D.C., I.K. STALEY, M. BAuMANN, R.P. RoTHMAN & W.L. HEARN. 1995. Identification of a primary metabolite of ibogaine that targets serotonin transporters and elevates serotonin. Pharmacol. Lett. 57: 45-50.
4. MASH, D.C., C.A. KOVERA, B.E. BucK, M.E. NoRENBERG, P. SHAPSHAK, W.L. HEARN & J. SANCHEZ-RAMos. 1998. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann. N. Y. Acad. Sci. 844: 274-292.
5. KLEIN, M. 1998. Research issues related to development of medications for treatment of cocaine addiction. Ann. N. Y. Acad. Sci. 844: 75-91.
6. MARKOU, A., T.R. KOSTEN & G.F. KOOB. 1998. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18(3): 136-174.
7. KOOB, G.F. & F.E. BLOOM. 1988. Cellular and molecular mechanisms of drug dependence. Science 242: 715-723.
8. MARKOU, A., F. WEISS, L.H. GOLD, S.B. CAINE, G. SCHULTEIS & G.F. KOOB. 1993. Animal models of drug craving. Psychopharmacology 1l2: 163-182.
9. HElM, M.H. & U.A. MEYER. 1990. Genotyping of poor metabolizers of debrisoquine by allele specific PCR amplification. Lancet 336: 529-532.
10. McLELLAN, A.T., H. KUSHNER, D. METZGER, R. PETERS, I. SMITH, G. GRISSOM, H. PETTINATI & M. ARTGERIOU. 1992. The fifth edition of the Addiction Severity Index. I. Subst. Abuse Treat. 9: 199-213.
11. SINGLETON, E.G. 1996. The HCQN-29: a short version of the Heroin Craving Questionnaire. Unpublished research. Available from the Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program NIDA, 55 Nathan Shock Drive, Baltimore, MD 21224, USA.
12. TIFFANY, S.T., E. SINGLETON, C.A. HAERTZEN & I.E. HENNINGFIELD. 1993. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 34: 19-28.
13. BECK, A.T., C.H. WARD, M. MENDELSON, I. MOCK & I. ERBAUGH. 1961. An interview for measuring depression. Arch. Gen. Psychiatry 4: 561-571.
14. OBACH, R.S., I. PABLO & D.C. MASH. 1998. Cytochrome P4502D6 catalyzes the 0-demethylation of the psychoactive alkaloid ibogaine to 12-hydroxyibogamine. Drug Metab. Dispos. 25(12): 1359-1369.
15. CARTER, B.L. & S. T. nFFANY. 1999. Meta-analysis of cue-reactivity in addiction research. Addiction 94(3): 327-340.
16. STALEY, I.K., Q. OUYANG, I. PABLO, W.L. HEARN, D.D. FLYNN, R.B. ROTHMAN, K.C. RICE & D.C. MASH. 1996. Pharmacological screen for activities of 12-hydroxyibogamine: a primary metabolite of the indole alkaloid ibogaine. Psychopharmacology 127: 10-18.
17. PABLO, I. & D.C. MASH. 1998. Noribogaine stimulates naloxone-sensitive (35S]GTPyS binding. Neuroreport 9:109-114.
18. MASH, D.C. & S. SCHENK. 1996. Preclinical screening of an ibogaine metabolite (noribogaine) on cocaine-induced hyperlocomotion and cocaine self-administration. Soc. Neurosci. Abstr. 22: 1929.
Monograph in PDF format: